Physicians: Discover the First and Only FDA-Approved Liquid Losartan
Arbli™ (losartan potassium) oral suspension 10 mg/mL delivers ready-to-dispense, tailored dosing for patients with oral adherence hurdles.
Prescribe Arbli™ with Confidence
Precision Dosing Meets Patient-Centered Care
Arbli, the first and only FDA-approved liquid losartan, eliminates the inconsistencies of compounded alternatives. Arbli bridges a critical treatment gap by offering the same proven efficacy and safety profile as tablet losartan but with flexible dosing for patients with dysphagia.
A Flexible Solution for Hypertension Care
- FDA-approved, first and only liquid losartan—interchangeable with losartan tablets
- Flexible, easy-to-tailor dosing compared to compounded losartan
- 24-month room-temperature stability, unopened—no refrigeration required
- Ready-to-dispense formulation eliminates compounding needs
More information for healthcare professionals:
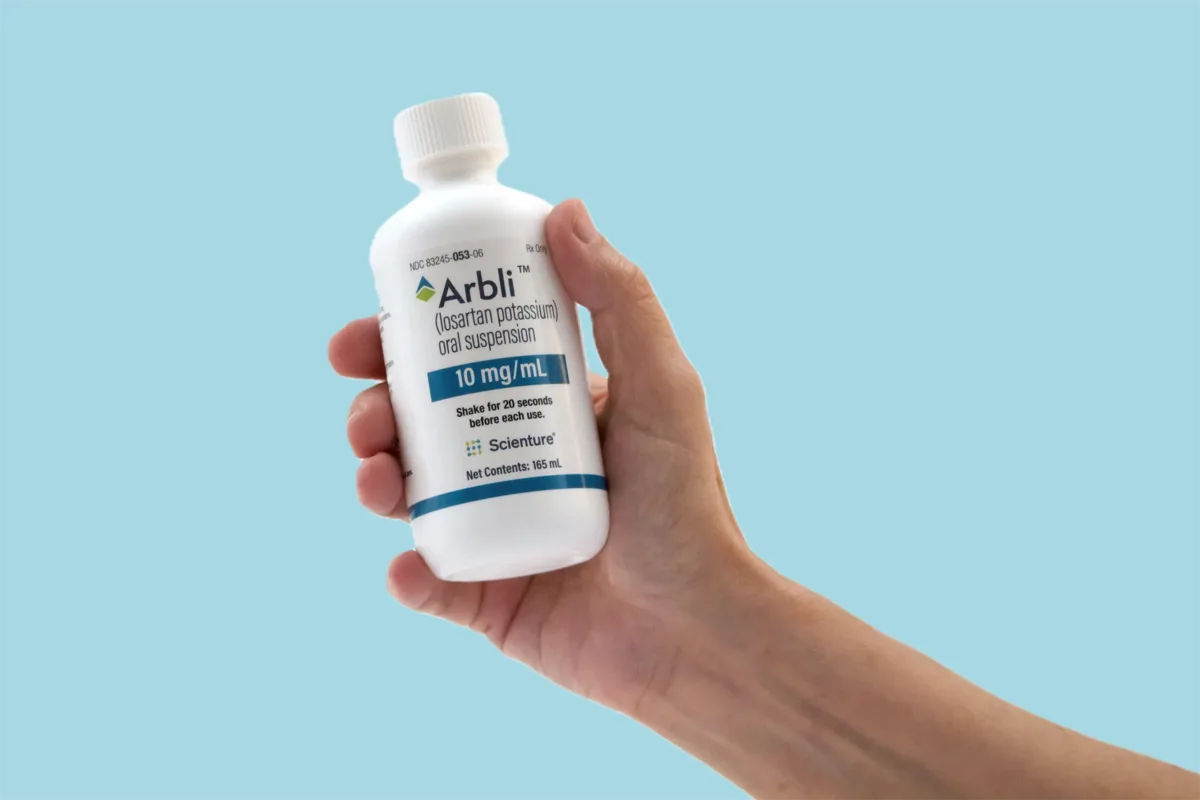
With reduced-volume dosing compared to compounded prescriptions, peppermint-flavored Arbli can support a smoother compliance journey, when conventional tablets or compounded losartan fall short.
Clinical Advantages
Meets Unique Patient Needs
- Provides a solution for patients with dysphagia or difficulty swallowing traditional 25 mg, 50 mg, and 100 mg tablets
- Reduces dosing volumes compared to compounded options—patients taking compounded losartan may receive up to four times the volume compared to Arbli
- FDA-approved formulation ensures safety, efficacy, and high-quality standards
Reduces Compounding Risks
Commercially available liquid formulation mitigates regulatory and quality risks associated with compounded preparations:
- FDA does not verify safety, effectiveness, or quality of compounded drugs
- Poor compounding practices can result in improper active pharmaceutical ingredient concentration
- Potential serious patient injury or death from compounding errors
Arbli is an angiotensin II receptor blocker (ARB) indicated for:1
- Treatment of hypertension to lower blood pressure in adults and children greater than 6 years old
- Reduction of stroke risk in patients with hypertension and left ventricular hypertrophy (not applicable to Black patients)
- Treatment of diabetic nephropathy with elevated serum creatinine and proteinuria in patients with type 2 diabetes and hypertension history

Arbli is indicated for children older than 6 years of age. Actor portrayals, not actual patients or caregivers.
Indicated for patients 6 years and older,1 this patented formulation can help address oral adherence challenges that may prevent patients from staying compliant with their chronic therapy.
Losartan Comparison Matrix
| Arbli oral suspension1 | Compounded losartan suspension2 | |
|---|---|---|
| Dose | 10 mg/mL | 2.5 mg/mL |
| Ready-to-dispense FDA-approved formulation | YES | NO* |
| Compliant with Good Manufacturing Practices | YES | Can vary† |
| Potency and Purity FDA Standards† | Meets | Can vary† |
| Refrigeration required | NO1 | YES2 |
| Active Pharmaceutical Ingredients (API) by FDA-approved sources | YES | Can vary |
| Shaking required | Shake for 20 seconds before dispensing dose1 | Shake each time before use‡ |
| Shelf life | Room-temperature for 60 days once opened§ | Once prepared, must be refrigerated, up to four weeks |
| At Retail Pharmacies | YES | Can vary |
*FDA-approved labels provide preparation for 2.5 mg/mL suspension.
†FDA does not verify the safety or effectiveness of compounded drugs before they are marketed.
‡Shake 2 minutes before prep, let stand one hour, then shake again for one minute. Must shake again for one minute after diluent mixture added. Shake each time before use.2
§Keep container tightly closed. Protect from light. Once the bottle is opened, use it within 60 days.1

Actor portrayal, not actual patient
Addressing Dysphagia
A Growing Clinical Challenge
Hypertension is an age-related disease state, with prevalence increasing markedly by 2030 as the U.S. population ages. Dysphagia presents a significant adherence barrier:
- 30% of community-dwelling older adults affected by dysphagia3
- 59% of aged care residents affected by dysphagia3
- 10-30% of aged care residents experience pill-specific dysphagia4
- 14% of community-dwelling older adults experience pill-specific dysphagia4
Impact on Patient Outcomes
Difficulty swallowing pills impacts medication adherence, leading to significant morbidity and mortality risks.5 With 67 million losartan prescriptions written annually, the patient population needing a liquid alternative could range from 670,000 to over 1 million prescriptions per year.

Actor portrayal, not actual patient
Formulation & Supply Information
- Concentration: 10 mg/mL oral suspension
- Shelf life: 24 months at room temperature
- To order: Contact Customer Service at 1-888-353-0221
Arbli allows for flexible dosing across various patient populations including those with difficulty swallowing tablets.
Prescribing considerations: Available in 165 mL bottles with child-resistant closure, facilitating precise dosing for pediatric patients, elderly patients with swallowing difficulties, and those requiring dose titration.
Institutional ordering: Available in 165 mL bottles and unit-dose packaging for institutional use; eligible for automatic refill programs through participating specialty pharmacies to ensure consistent patient access.
Inventory and logistics: Stocked at major wholesalers; available through both retail and specialty pharmacies to accommodate various insurance requirements and patient care settings.
Helpful Information for Prescribers
References
1. ARBLITM [prescribing information]. Commack, NY: Scienture, LLC. 2025
2. COZAAR® label. Accessed on dailymed.nlm.nih.gov. COZAAR® label provides suspension is 2.5 mg/mL in 200 mL solution.
3. Thanh-Nhan D, Wen-Chao H, Liang-Hui W, Fei-Chun C, Nguyen TN, Li-Wei C. Prevalence and methods for assessment of oropharyngeal dysphagia in older adults: a systematic review and meta analysis. J Clin Med. 2022;11(9):2605. https://doi.org/10.3390/ jcm11092605
4. Mc Gillicuddy A, Crean AM, Sahm LJ. Older adults with difficulty swallowing oral medicines: a systematic review of the literature. Eur J Clin Pharmacol. 2016;72:141–51. https://doi.org/10.1007/s00228-015-1979-8
5. Sefidani Forough A, Lau ET, Steadman KJ, Cichero JA, Kyle GJ, Serrano Santos JM, Nissen LM. A spoonful of sugar helps the medicine go down? A review of strategies for ma king pills easier to swallow. Patient Prefer Adherence. 2018;1337–46. https://doi.org/10.2147/ppa.S164406
COZAAR® is a registered trademark of N.V. Organon.

